ammonia boiling point
Boiling point of Argon-186. Answer 1 of 3.

Why Is Boiling Point Of Ammonia Greater Than Arsine Chemistry Stack Exchange
Its boiling point is 3335 C 2803 F and its freezing point is 777 C 1078 F.
. 091 gmL at 20 C. Ammonia Boiling point 1013 bar -335 o C Ammonia Latent heat of vaporization 1013 bar at boiling point. To store it in large quantities it is either liquefied under pressure about 10 bar at 25 C or is refrigerated boiling point 33 C. Water H2O has a mass of 18 and makes hydrogen bonds.
NH3 boils at 3334 C 28012 F at a pressure of one atmosphere. When heated above its critical temperature of 2703 F ammonia exists only as a vapor regardless of the pressure. The melting and boiling points of molecular substances are determined largely by the intermolecular forces. Boiling point of water.
56 C 1328 F Boiling point of alcohol. Boiling point of Anisole. The freezing point of a 26 Baumé solution is about -110F. NH 3 PER CU.
149 rows weight of liquid ammonia 515 pounds per gallon water weight 833 pounds per. Boiling point of fluoride is 195 degrees Celsius while boiling point of ammonia is minus 33 degrees Celsius which makes 535 degrees difference. -1958 C -3204 F. The freezing point of other concentrations is in the table below.
Ammonia boils at -28 F and fre ezes to a white crystalline mass at -108 F. Ammonia NH3 has a mass of 17 and makes hydrogen bonds. At room temperature ammonia is a colorless highly irritating gas with a pungent suffocating odor. Boiling point of Antimony.
Boiling point of Ammonia-355. Boiling Point The boiling point of aqua ammonia is defined as the temperature at which the partial vapor pressure of the ammonia vapor over the aqua ammonia equals atmospheric pressure. Ammonia is a colourless gas with a sharp penetrating odour. Hazardous Substances Data Bank HSDB Ammonia is manufactured primarily by a modified Haber reduction process using atmospheric nitrogen and a hydrogen source for example methane ethylene or naphtha at high temperatures 400 to 6500 C and pressures 100 to 900 atm in the presence of an iron catalyst.
Boiling point of Aniline. What is the boiling point of ammonia. Boiling points of common materials. 13712 kJkg Ammonia Vapor pressure at 21 o C or 70 o F.
7837 C 1731 F Boiling point of nitrogen. Since ammonia features hydrogen bonding owing to the presence of covalent bonds between hydrogen and nitrogen which is relatively more electronegative and has a lone pair it has a higher boiling point than phosphine. 72K views View upvotes. 647 C 1485 F Boiling point of acetone.
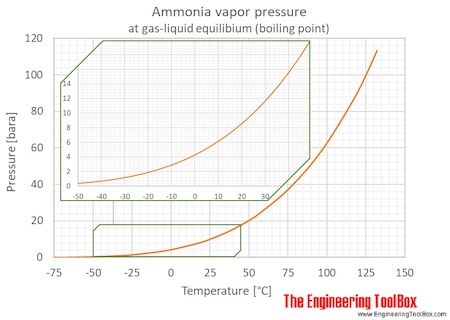
It should be noted however that if liquid ammonia is spilled or released to the atmosphere at normal temperatures the resultant pool of boiling liquid will be significantly colder than 28F. -75 C 008 bar bar upper limit. Between the melting and critical points liquid ammonia exerts a vapor pressure which increases with rising temperature. As mentioned before apart from being moderately explosive when mixed with air ammonia is toxic.
Lower limit for calculation. Ammonias boiling point is 133 degrees CelsiusKelvin lower then that of water. At atmospheric pressure a closed container of ammonia vapor and liquid will be in equilibrium at a temperature of 28F 33C. Boiling point of Argon.
100 C 212 F Boiling point of water in Kelvin. Their masses are 17 amu and 20 amu respectively difference is very small. The 3d structure may be viewed using Java or Javascript. -2801F -3334C Is ammonia a chemical property.
At normal ambient conditions it is a gas. Readily than water. In pure form it is known as anhydrous ammonia and is hygroscopic readily absorbs moisture. Use this link for bookmarking this species for future reference.
The ammonia molecule has a trigonal pyramidal shape as predicted by the valence shell electron pair repulsion theory VSEPR theory with an experimentally determined. 6060F NH 3 BY WT. SPECIFIC GRAVITY BOILING POINTS AND FREEZING POINTS OF VARIOUS AQUA AMMONIA SOLUTIONS AMMONIA CONCENTRATION BAUMÉ 60 F SP. It can be contained in tanks of carbon steel or CrNi Mo steel.
So I know the forces responsible for the boiling point are the vanderwale forces depending on the mass of the molecules and the hydrogen bonds. It has a high heat of vaporization 233 kilojoules per mole at its boiling point and can be handled as a liquid in thermally insulated containers in the laboratory. So water needs more heat energy and is more tolerant to heat than ammonia. Degrees Be Weight NH3 Boiling Point at 60 Concentration F 10 000 212 11 162 195 12 330 186 13 502 177 14 674 171 15 849 163 16 1028 156 17 1210 149 18 1396 142.
Calculation of thermodynamic state variables of ammonia on the boiling curve. Aqueous ammonia CAS Number. Ammonia has pretty strong intermolecular forces because it can form hydrogen bonds however it cant form as many hydrogen bonds per molecule as water and so its boiling point and melting point are lower than waters. 130 C 108 bar.
Ammonia has a boiling point of -33 degrees while the boiling point of water is 100 degree Celsius. Permanent link for this species. Some chemicalphysical properties of ammonia are. 7837 C 1731 F Boiling point of methanol.
3732 K Boiling point of ethanol.

Ammonia I Nh Sub 3 Sub I Thermodynamic Properties

Physical Properties Of Ammonia Download Scientific Diagram

Liquid Ammonia Thermal Properties At Saturation Pressure


Posting Komentar untuk "ammonia boiling point"